In this article, you will learn Internal Validity vs External Validity in research with the help of examples.
When conducting research, it is important to be aware of the difference between internal and external validity. Internal validity refers to the extent to which the results of a study can be generalized to the population of interest. External validity refers to the extent to which the results of a study can be generalized to other populations.
Internal Validity
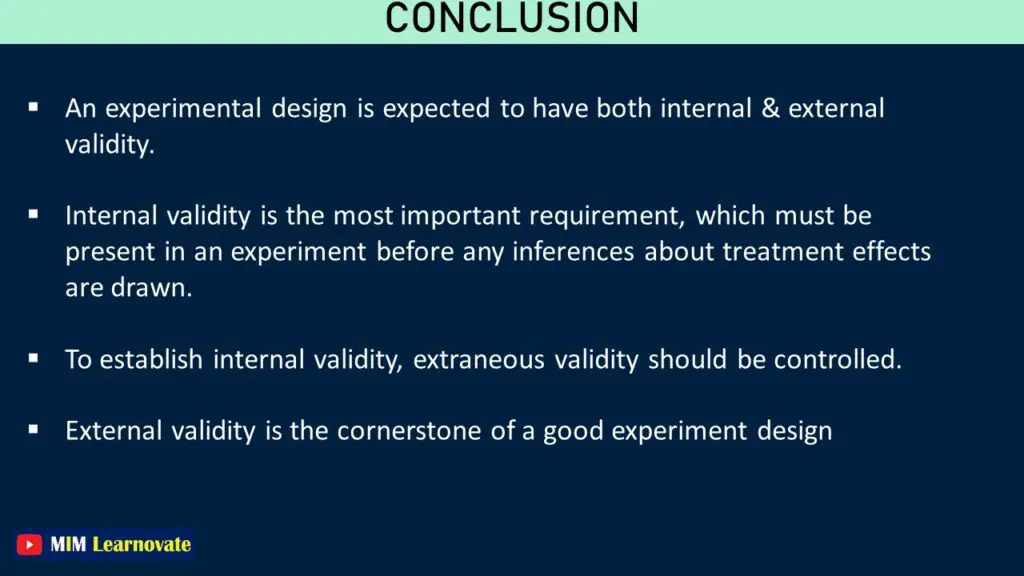
Internal validity is the term used in statistics to describe the accuracy measure that examines the validity of the experiment, particularly with reference to confounding. It determines whether or not the independent factors are to accountable for the observed effects on the dependent variables. Internal validity is the degree to which a study’s results can be generalized to the population of interest. Studies with high internal validity are able to draw causal conclusions about relationships between variables.
It is challenging to draw reliable conclusions regarding the relationship between variables when observable effects are influenced by or confused by auxiliary factors.
Internal validity is simply the level of justification for a cause-and-effect and effect relationship based on an experiment, determined by how well the experiment avoids systematic errors.
Because it overlooks confounding factors, high internal validity enables the researcher to select one explanation over another with sufficient confidence An experiment’s internal validity increases as confounding levels decrease.
Example of Internal Validity
If a study is investigating the effects of a new medication on anxiety, internal validity would be concerned with whether the observed effects are actually due to the medication and not some other factor.
A researcher developed a hypothesis that word puzzle apps will help to prevent negative thinking.
To test the hypothesis, the researcher randomly recruited and evenly divided people into two groups. There are two groups: ✔the treatment group (those who will use the app) ✔ the control group.
In addition to allocating participants at random, the researcher ensured that the participants were uninformed of the research objectives and the other test group.

External Validity
The definition of “external validity” includes assessing the generalizability of the casual relationship found in the study.
By assessing how well the research findings may be used in different contexts, external validity establishes the correctness of the findings.
External validity is concerned with the extent to which the results of a study can be generalized to other people and situations.
The most important determinant of external validity is the similarity between the population or situation being studied and the population or situation to which the results are being generalized. If there is little similarity, then it is unlikely that the results will be applicable.
External validity is at threat when the particular collection of research settings does not actually account for the interaction of other real-world circumstances.
Example of External Validity
For example, if a study is conducted on college students, it would not have high external validity for elementary school children. Likewise, if a study is conducted in a laboratory, it would not have high external validity for real-world settings.
Internal Validity vs External Validity
The following points describe the distinctions between internal and external validity:
1- Internal Validity is just a measure of the experiment’s accuracy. External validity, on the other hand, investigates whether or not the cause-and-effect relationship discovered in the experiment may be generalized.

2- Internal validity is defined as the amount to which the experiment is error-free and any discrepancy in measurement is related to an independent variable and nothing else. External validity is the extent to which research findings can be extended to the rest of the world.
3- External validity determines whether or not the casual relationship revealed in the experiment may be generalized. Internal validity is concerned with extraneous control variable .
.

4- Internal validity measures the strength of the study techniques and design. External validity, on the other hand, investigates the generalizability of research findings to the real world.

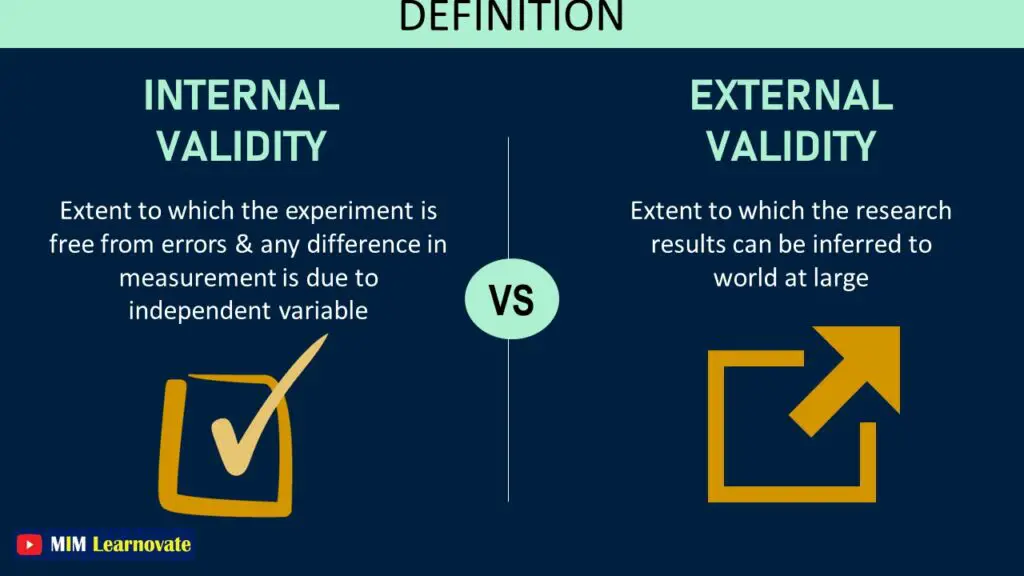
5- Internal validity indicates how well the conclusion is supported. In contrast, external validity determines the extent to which the study is justified in generalizing to another setting.
6- Internal validity considers or excludes alternate explanations for the outcome. External validity, on the other hand, is utilized to generalize the outcome.

Difference between Internal Validity and External Validity
| Internal Validity | External Validity | |
| Definition | Degree to which the experiment is error-free and any measurement difference is directly due to the independent variable. | Degree to which the research results can be applied to the general public. |
| What it is? | A measure of an experiment’s accuracy. | Examines whether or not the casual relationship found during the experiment can be applied generally. |
| Concerned with | Concerned with control, of extraneous variable. | Emphasizes how the results can be used in real-world contexts. |
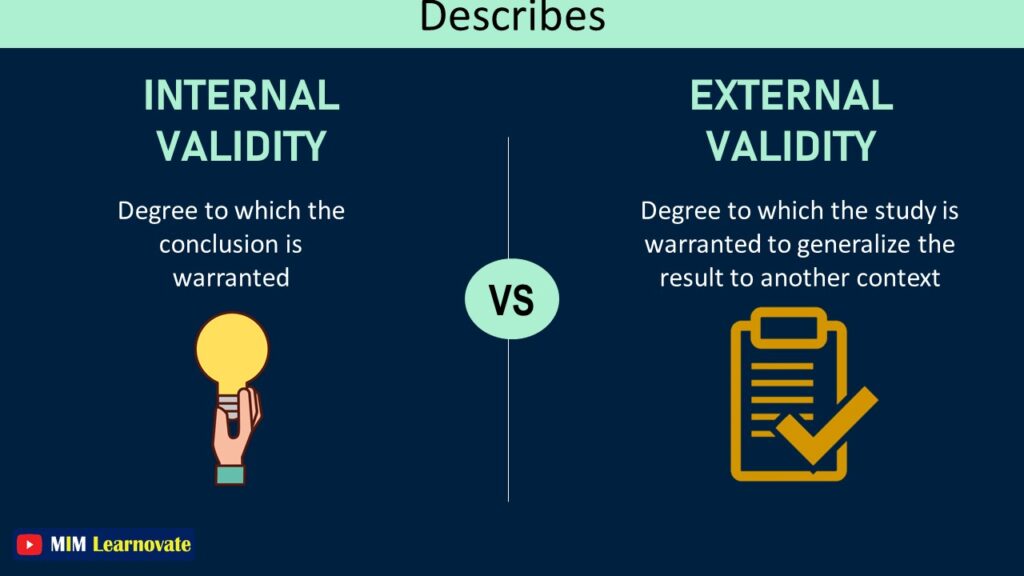
| Describes | Refers to how strongly a conclusion is supported. | Determines the extent to which the study is justified in extending the results to other contexts. |
| Identifies | Evaluates the strength of the research design and methods. | Indicates how applicable the study’s findings are in the real world. |
| Used to | Alternative explanations for the finding are either addressed or eliminated. | Used to generalize the results. |
Similarities between Internal Validity and External Validity
✔ Both of these factors should be taken into account when planning a study since they affect whether the findings are meaningful.
✔ Researchers always assess how well their study performs in terms of both types of validity.
Usually, a research article that is published in an academic journal reports on each of these ideas. This is done so that other researchers may assess the study and determine whether the findings are reliable and valid.
Trade-off between Internal and External Validity
When designing research, scientists face a trade-off between internal and external validity.
External validity frequently suffers as a result of improved internal validity (and vice versa). Your research priorities are reflected in the type of study you select.
Studies with high internal validity are often small and not representative of the general population, while studies with high external validity are often large and not able to measure specific variables.
Trade-off Example
A causal relationship can be examined in a simulated lab environment or in the ‘real world’. Because external influences can be reduced in a laboratory environment, stronger internal validity is guaranteed. However, because a lab environment is different from the “real world,” the external validity is reduced (that does have external influencing factors).
This trade-off can be resolved by conducting the research first in a controlled (artificial) environment to determine the existence of a causal relationship, then in the field to determine whether the findings hold true in the actual world.
Conclusion
✔ An experimental design should have both internal and external validity.
✔ Prior to drawing conclusions regarding treatment effects from an experiment, internal validity is the most crucial criteria that must be met.
✔ Controlling extraneous validity is necessary to establish internal validity.
✔ However, external validity—the key component of a successful experiment design—is challenging to achieve.